Oliver, G. Lymphatic vasculature development. Nat. Rev. Immunol. 4, 35–45 (2004).
Google Scholar
Oliver, G. & Alitalo, K. The lymphatic vasculature: recent progress and paradigms. Annu Rev. Cell Dev. Biol. 21, 457–483 (2005).
Google Scholar
Petrova, T. V. & Koh, G. Y. Organ-specific lymphatic vasculature: From development to pathophysiology. J. Exp. Med. 215, 35–49 (2018).
Google Scholar
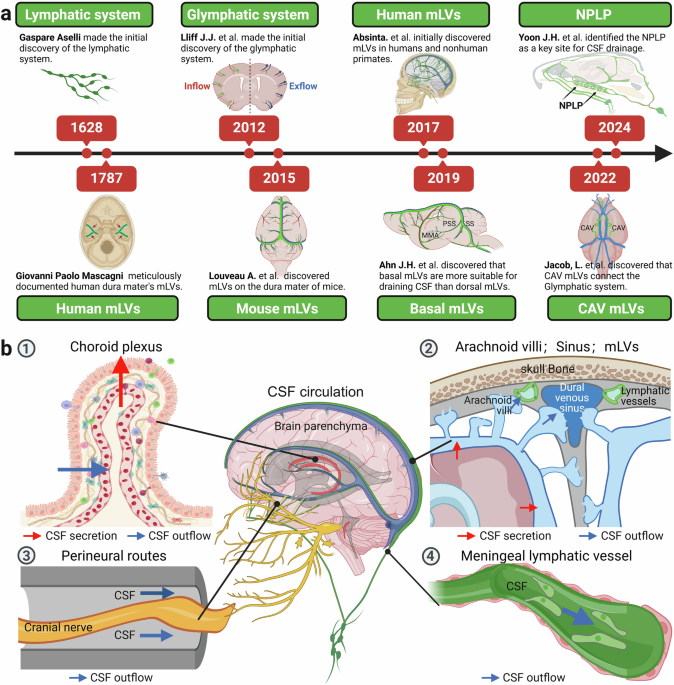
G, A. De lactibus, sive lacteis venis, quarto vasorum mesaraicorum genere, novo invento Gasparis Asellii Cremo. Dissertatio. (MDCXXIIX), Milan, (1628).
Wilting, J. & Becker, J. The lymphatic vascular system: much more than just a sewer. Cell Biosci. 12, 157 (2022).
Google Scholar
Mascagni, P., & Ciro, S. Vasorum lymphaticorum Corporis Humani Historia et Ichnographia (Ex typographia Pazzini Carli, 1787).
Sandrone, S., Moreno-Zambrano, D., Kipnis, J. & van Gijn, J. A (delayed) history of the brain lymphatic system. Nat. Med. 25, 538–540 (2019).
Google Scholar
Lecco, V. [Probable modification of the lymphatic fissures of the walls of the venous sinuses of the dura mater]. Arch. Ital. Otol. Rinol. Laringol. 64, 287–296 (1953).
Google Scholar
Foldi, M. et al. New contributions to the anatomical connections of the brain and the lymphatic system. Acta Anat. 64, 498–505 (1966).
Google Scholar
Andres, K. H., von During, M., Muszynski, K. & Schmidt, R. F. Nerve fibres and their terminals of the dura mater encephali of the rat. Anat. Embryol. 175, 289–301 (1987).
Google Scholar
Zakharov, A., Papaiconomou, C. & Johnston, M. Lymphatic vessels gain access to cerebrospinal fluid through unique association with olfactory nerves. Lymphat Res. Biol. 2, 139–146 (2004).
Google Scholar
Gausas, R. E., Daly, T. & Fogt, F. D2-40 expression demonstrates lymphatic vessel characteristics in the dural portion of the optic nerve sheath. Ophthalmic Plast. Reconstr. Surg. 23, 32–36 (2007).
Google Scholar
Wichmann, T. O., Damkier, H. H. & Pedersen, M. A Brief Overview of the Cerebrospinal Fluid System and Its Implications for Brain and Spinal Cord Diseases. Front. Hum. Neurosci. 15, 737217 (2021).
Google Scholar
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 4, 147ra111 (2012).
Google Scholar
Brinker, T., Stopa, E., Morrison, J. & Klinge, P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 11, 10 (2014).
Google Scholar
Aspelund, A. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212, 991–999 (2015).
Google Scholar
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Google Scholar
Koh, L., Zakharov, A. & Johnston, M. Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res. 2, 6 (2005).
Google Scholar
Sadegh, C. et al. Choroid plexus-targeted NKCC1 overexpression to treat post-hemorrhagic hydrocephalus. Neuron 111, e1594 (2023).
Google Scholar
Xu, H. et al. Choroid plexus NKCC1 mediates cerebrospinal fluid clearance during mouse early postnatal development. Nat. Commun. 12, 447 (2021).
Google Scholar
Proulx, S. T. Cerebrospinal fluid outflow: a review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell Mol. Life Sci. 78, 2429–2457 (2021).
Google Scholar
Zhou, Y. et al. Impaired peri-olfactory cerebrospinal fluid clearance is associated with ageing, cognitive decline and dyssomnia. EBioMedicine 86, 104381 (2022).
Google Scholar
Ma, Q., Ineichen, B. V., Detmar, M. & Proulx, S. T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 8, 1434 (2017).
Google Scholar
Cserr, H. F., Harling-Berg, C. J. & Knopf, P. M. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 2, 269–276 (1992).
Google Scholar
Cheng, Y. & Wang, Y. J. Meningeal Lymphatic Vessels: A Drain of the Brain Involved in Neurodegeneration? Neurosci. Bull. 36, 557–560 (2020).
Google Scholar
Decker, Y. et al. Magnetic resonance imaging of cerebrospinal fluid outflow after low-rate lateral ventricle infusion in mice. JCI Insight. 7, e150881 (2022).
Liu, G. et al. Direct Measurement of Cerebrospinal Fluid Production in Mice. Cell Rep. 33, 108524 (2020).
Google Scholar
Wen, Y. R., Yang, J. H., Wang, X. & Yao, Z. B. Induced dural lymphangiogenesis facilities soluble amyloid-beta clearance from brain in a transgenic mouse model of Alzheimer’s disease. Neural Regen. Res. 13, 709–716 (2018).
Google Scholar
Tarasoff-Conway, J. M. et al. Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 11, 457–470 (2015).
Google Scholar
Chiu, C. et al. Temporal course of cerebrospinal fluid dynamics and amyloid accumulation in the aging rat brain from three to thirty months. Fluids Barriers CNS 9, 3 (2012).
Google Scholar
Mestre, H. et al. Periarteriolar spaces modulate cerebrospinal fluid transport into brain and demonstrate altered morphology in aging and Alzheimer’s disease. Nat. Commun. 13, 3897 (2022).
Google Scholar
Zamani, A. et al. Impaired glymphatic function in the early stages of disease in a TDP-43 mouse model of amyotrophic lateral sclerosis. Transl. Neurodegener. 11, 17 (2022).
Google Scholar
Eisen, A., Nedergaard, M., Gray, E. & Kiernan, M. C. The glymphatic system and Amyotrophic lateral sclerosis. Prog. Neurobiol. 234, 102571 (2024).
Google Scholar
Liu, S. et al. Glymphatic dysfunction in patients with early-stage amyotrophic lateral sclerosis. Brain 147, 100–108 (2024).
Google Scholar
Ma, Q. et al. Clearance of cerebrospinal fluid from the sacral spine through lymphatic vessels. J. Exp. Med. 216, 2492–2502 (2019).
Google Scholar
Li, Q. et al. Drainage of senescent astrocytes from brain via meningeal lymphatic routes. Brain Behav. Immun. 103, 85–96 (2022).
Google Scholar
Ding, X. B. et al. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease. Nat. Med. 27, 411–418 (2021).
Google Scholar
Liu, Z. et al. The cervical lymph node contributes to peripheral inflammation related to Parkinson’s disease. J. Neuroinflammation 20, 93 (2023).
Google Scholar
Da Mesquita, S. et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560, 185–191 (2018).
Google Scholar
Patel, T. K. et al. Dural lymphatics regulate clearance of extracellular tau from the CNS. Mol. Neurodegener. 14, 11 (2019).
Google Scholar
Bolte, A. C. et al. Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. Nat. Commun. 11, 4524 (2020).
Google Scholar
Liao, J. et al. Improving the Function of Meningeal Lymphatic Vessels to Promote Brain Edema Absorption after Traumatic Brain Injury. J. Neurotrauma 40, 383–394 (2023).
Google Scholar
Liu, M. et al. Exogenous interleukin 33 enhances the brain’s lymphatic drainage and toxic protein clearance in acute traumatic brain injury mice. Acta Neuropathol. Commun. 11, 61 (2023).
Google Scholar
Eide, P. K. & Ringstad, G. Glymphatic-stagnated edema induced by traumatic brain injury. Trends Pharm. Sci. 45, 388–390 (2024).
Google Scholar
Hussain, R. et al. Potentiating glymphatic drainage minimizes post-traumatic cerebral oedema. Nature 623, 992–1000 (2023).
Google Scholar
Zhang, Q. et al. Neutrophil extracellular trap-mediated impairment of meningeal lymphatic drainage exacerbates secondary hydrocephalus after intraventricular hemorrhage. Theranostics 14, 1909–1938 (2024).
Google Scholar
Yuan, J. et al. Inactivation of ERK1/2 signaling mediates dysfunction of basal meningeal lymphatic vessels in experimental subdural hematoma. Theranostics 14, 304–323 (2024).
Google Scholar
Yang, L. et al. Blocking cerebral lymphatic system reduces central and peripheral inflammatory response in ischemic stroke. Brain Res. 1831, 148825 (2024).
Google Scholar
Chen, Y. et al. Vitamin D accelerates the subdural hematoma clearance through improving the meningeal lymphatic vessel function. Mol. Cell Biochem 479, 3129–3140 (2024).
Google Scholar
Zhang, J. et al. The Drainage Dysfunction of Meningeal Lymphatic Vessels Is Correlated with the Recurrence of Chronic Subdural Hematoma: a Prospective Study. Transl. Stroke Res. (2023).
Wang, X. et al. Single-Cell RNA Sequencing and Spatial Transcriptomics Reveal Pathogenesis of Meningeal Lymphatic Dysfunction after Experimental Subarachnoid Hemorrhage. Adv. Sci. 10, e2301428 (2023).
Google Scholar
Li, D. et al. Photostimulation of brain lymphatics in male newborn and adult rodents for therapy of intraventricular hemorrhage. Nat. Commun. 14, 6104 (2023).
Google Scholar
Wang, X. et al. Dobutamine promotes the clearance of erythrocytes from the brain to cervical lymph nodes after subarachnoid hemorrhage in mice. Front. Pharm. 13, 1061457 (2022).
Google Scholar
Tsai, H. H. et al. Functional Investigation of Meningeal Lymphatic System in Experimental Intracerebral Hemorrhage. Stroke 53, 987–998 (2022).
Google Scholar
Bai, S. et al. Cranial Bone Transport Promotes Angiogenesis, Neurogenesis, and Modulates Meningeal Lymphatic Function in Middle Cerebral Artery Occlusion Rats. Stroke 53, 1373–1385 (2022).
Google Scholar
Liu, X. et al. Subdural haematomas drain into the extracranial lymphatic system through the meningeal lymphatic vessels. Acta Neuropathol. Commun. 8, 16 (2020).
Google Scholar
Kovacs, M. A. et al. Vascular Endothelial Growth Factor-C Treatment Enhances Cerebrospinal Fluid Outflow during Toxoplasma gondii Brain Infection but Does Not Improve Cerebral Edema. Am. J. Pathol. 194, 225–237 (2024).
Google Scholar
Feng, J. et al. Impaired meningeal lymphatic drainage in Listeria monocytogenes infection. Front. Immunol. 15, 1382971 (2024).
Google Scholar
Dong, H. et al. Enhanced meningeal lymphatic drainage ameliorates lipopolysaccharide-induced brain injury in aged mice. J. Neuroinflammation 21, 36 (2024).
Google Scholar
Huang, W. et al. Histopathological changes of the dural myeloid cells and lymphatic vessels in a mouse model of sepsis-associated encephalopathy. Exp. Neurol. 369, 114521 (2023).
Google Scholar
Lempriere, S. Meningeal lymphatics mediate drainage of viruses from the CNS. Nat. Rev. Neurol. 18, 382 (2022).
Google Scholar
Li, X. et al. Meningeal lymphatic vessels mediate neurotropic viral drainage from the central nervous system. Nat. Neurosci. 25, 577–587 (2022).
Google Scholar
Song, E. et al. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 577, 689–694 (2020).
Google Scholar
Hu, X. et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. 30, 229–243 (2020).
Google Scholar
Zhou, C. et al. Meningeal lymphatics regulate radiotherapy efficacy through modulating anti-tumor immunity. Cell Res. 32, 543–554 (2022).
Google Scholar
Zhou, C., Xu, H. & Luo, J. Meningeal lymphatic vasculature, a general target for glioblastoma therapy? Fundam. Res. 4, 267–269 (2024).
Google Scholar
Wang, M. et al. Disturbed meningeal lymphatic function associated with malignancy and progression in patients with intracranial malignant tumors. Med 4, e894 (2023).
Google Scholar
Wu, C. H. et al. Impaired Glymphatic and Meningeal Lymphatic Functions in Patients with Chronic Migraine. Ann. Neurol. 95, 583–595 (2024).
Google Scholar
Peng, T. et al. The cerebral lymphatic drainage system and its implications in epilepsy. J. Neurosci. Res. 102, e25267 (2024).
Google Scholar
Baluk, P. et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 204, 2349–2362 (2007).
Google Scholar
Yamaguchi, S. et al. The development of early human lymphatic vessels as characterized by lymphatic endothelial markers. EMBO J. 43, 868–885 (2024).
Google Scholar
Tammela, T. & Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 140, 460–476 (2010).
Google Scholar
Aspelund, A. et al. Lymphatic System in Cardiovascular Medicine. Circ. Res. 118, 515–530 (2016).
Google Scholar
Srinivasan, R. S. et al. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 21, 2422–2432 (2007).
Google Scholar
Antila, S. et al. Development and plasticity of meningeal lymphatic vessels. J. Exp. Med. 214, 3645–3667 (2017).
Google Scholar
Margaris, K. N. & Black, R. A. Modelling the lymphatic system: challenges and opportunities. J. R. Soc. Interface 9, 601–612 (2012).
Google Scholar
Brakenhielm, E. & Alitalo, K. Cardiac lymphatics in health and disease. Nat. Rev. Cardiol. 16, 56–68 (2019).
Google Scholar
Ahn, J. H. et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572, 62–66 (2019).
Google Scholar
Smyth, L. C. D. et al. Identification of direct connections between the dura and the brain. Nature 627, 165–173 (2024).
Google Scholar
Jacob, L. et al. Conserved meningeal lymphatic drainage circuits in mice and humans. J Exp Med. 219, e20220035 (2022).
Norwood, J. N. et al. Anatomical basis and physiological role of cerebrospinal fluid transport through the murine cribriform plate. Elife. 8, e44278 (2019).
Yoon, J. H. et al. Nasopharyngeal lymphatic plexus is a hub for cerebrospinal fluid drainage. Nature 625, 768–777 (2024).
Google Scholar
Vera Quesada, C. L., Rao, S. B., Torp, R. & Eide, P. K. Widespread distribution of lymphatic vessels in human dura mater remote from sinus veins. Front Cell Dev. Biol. 11, 1228344 (2023).
Google Scholar
Vera Quesada, C. L., Rao, S. B., Torp, R. & Eide, P. K. Immunohistochemical visualization of lymphatic vessels in human dura mater: methodological perspectives. Fluids Barriers CNS 20, 23 (2023).
Google Scholar
Jayatilaka, A. D. An electron microscopic study of sheep arachnoid granulations. J. Anat. 99, 635–649 (1965).
Google Scholar
Absinta, M. et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife. 6, e29738 (2017).
Shah, T. et al. Arachnoid granulations are lymphatic conduits that communicate with bone marrow and dura-arachnoid stroma. J. Exp. Med. 220, e20220618 (2023).
Scallan, J. P., Zawieja, S. D., Castorena-Gonzalez, J. A. & Davis, M. J. Lymphatic pumping: mechanics, mechanisms and malfunction. J. Physiol. 594, 5749–5768 (2016).
Google Scholar
Yin, X. et al. Compartmentalized ocular lymphatic system mediates eye-brain immunity. Nature 628, 204–211 (2024).
Google Scholar
Delle, C., Wang, X. & Nedergaard, M. The Ocular Glymphatic System-Current Understanding and Future Perspectives. Int. J. Mol. Sci. 25, 5734 (2024).
Mazzitelli, J. A. et al. Cerebrospinal fluid regulates skull bone marrow niches via direct access through dural channels. Nat. Neurosci. 25, 555–560 (2022).
Google Scholar
Sevick-Muraca, E. M., Kwon, S. & Rasmussen, J. C. Emerging lymphatic imaging technologies for mouse and man. J. Clin. Invest 124, 905–914 (2014).
Google Scholar
Proulx, S. T. et al. Expansion of the lymphatic vasculature in cancer and inflammation: new opportunities for in vivo imaging and drug delivery. J. Control Rel. 172, 550–557 (2013).
Google Scholar
Munn, L. L. & Padera, T. P. Imaging the lymphatic system. Microvasc. Res. 96, 55–63 (2014).
Google Scholar
Gruber-Rouh, T. et al. Direct lymphangiography as treatment option of lymphatic leakage: indications, outcomes and role in patient’s management. Eur. J. Radio. 83, 2167–2171 (2014).
Google Scholar
Johnson, O. W. et al. The thoracic duct: clinical importance, anatomic variation, imaging, and embolization. Eur. Radio. 26, 2482–2493 (2016).
Google Scholar
Toliyat, M. et al. Interventional radiology in the management of thoracic duct injuries: Anatomy, techniques and results. Clin. Imaging 42, 183–192 (2017).
Google Scholar
Lambertz, R. et al. Ultrasound-guided lymphangiography and interventional embolization of chylous leaks following esophagectomy. Innov. Surg. Sci. 4, 85–90 (2019).
Google Scholar
Sajedi, S., Sabet, H. & Choi, H. S. Intraoperative biophotonic imaging systems for image-guided interventions. Nanophotonics 8, 99–116 (2019).
Google Scholar
Hellingman, D. et al. A New Portable Hybrid Camera for Fused Optical and Scintigraphic Imaging: First Clinical Experiences. Clin. Nucl. Med. 41, e39–e43 (2016).
Google Scholar
Surasi, D. S., O’Malley, J. & Bhambhvani, P. 99 mTc-Tilmanocept: A Novel Molecular Agent for Lymphatic Mapping and Sentinel Lymph Node Localization. J. Nucl. Med. Technol. 43, 87–91 (2015).
Google Scholar
Simanek, M. & Koranda, P. SPECT/CT imaging in breast cancer – current status and challenges. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 160, 474–483 (2016).
Google Scholar
Tew, K. & Farlow, D. SPECT/CT in Melanoma Lymphoscintigraphy. Clin. Nucl. Med. 41, 961–963 (2016).
Google Scholar
Koyyalamudi, R. T. & Rossleigh, M. A. Lymphoscintigraphic SPECT/CT-Contralateral Axillary Sentinel Lymph Node Drainage in Breast Cancer. Clin. Nucl. Med. 42, 121–122 (2017).
Google Scholar
Saad, Z. Z. et al. Investigating the role of SPECT/CT in dynamic sentinel lymph node biopsy for penile cancers. Eur. J. Nucl. Med. Mol. Imaging 44, 1176–1184 (2017).
Google Scholar
Kitai, T., Inomoto, T., Miwa, M. & Shikayama, T. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer 12, 211–215 (2005).
Google Scholar
Unno, N. et al. Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. J. Vasc. Surg. 45, 1016–1021 (2007).
Google Scholar
Sevick-Muraca, E. M. et al. Imaging of lymph flow in breast cancer patients after microdose administration of a near-infrared fluorophore: feasibility study. Radiology 246, 734–741 (2008).
Google Scholar
Rockson, S. G. A Role for Near Infrared Fluorescent Imaging in the Evaluation of Lymphatic Function. Lymphat Res. Biol. 15, 203 (2017).
Google Scholar
Zeltzer, A. A. et al. MR lymphography in patients with upper limb lymphedema: The GPS for feasibility and surgical planning for lympho-venous bypass. J. Surg. Oncol. 118, 407–415 (2018).
Google Scholar
Neligan, P. C., Kung, T. A. & Maki, J. H. MR lymphangiography in the treatment of lymphedema. J. Surg. Oncol. 115, 18–22 (2017).
Google Scholar
Kajita, H. et al. Photoacoustic lymphangiography. J. Surg. Oncol. 121, 48–50 (2020).
Google Scholar
Soergel, P. et al. Sentinel Lymphadenectomy in Vulvar Cancer Using Near-Infrared Fluorescence From Indocyanine Green Compared With Technetium 99 m Nanocolloid. Int. J. Gynecol. Cancer 27, 805–812 (2017).
Google Scholar
Pavlista, D. & Eliska, O. Analysis of direct oil contrast lymphography of upper limb lymphatics traversing the axilla – a lesson from the past – contribution to the concept of axillary reverse mapping. Eur. J. Surg. Oncol. 38, 390–394 (2012).
Google Scholar
Mellor, R. H. et al. Lymphatic dysfunction, not aplasia, underlies Milroy disease. Microcirculation 17, 281–296 (2010).
Google Scholar
Pappalardo, M. & Cheng, M. H. Lymphoscintigraphy for the diagnosis of extremity lymphedema: Current controversies regarding protocol, interpretation, and clinical application. J. Surg. Oncol. 121, 37–47 (2020).
Google Scholar
Moncayo, V. M., Aarsvold, J. N. & Alazraki, N. P. Lymphoscintigraphy and sentinel nodes. J. Nucl. Med. 56, 901–907 (2015).
Google Scholar
Naaman, Y. et al. The Added Value of SPECT/CT in Sentinel Lymph Nodes Mapping for Endometrial Carcinoma. Ann. Surg. Oncol. 23, 450–455 (2016).
Google Scholar
Iimura, T. et al. Estimating Lymphodynamic Conditions and Lymphovenous Anastomosis Efficacy Using (99m)Tc-phytate Lymphoscintigraphy with SPECT-CT in Patients with Lower-limb Lymphedema. Plast. Reconstr. Surg. Glob. Open 3, e404 (2015).
Google Scholar
Mazzei, M. A. et al. High-resolution MR lymphangiography for planning lymphaticovenous anastomosis treatment: a single-centre experience. Radio. Med. 122, 918–927 (2017).
Google Scholar
Kajita, H. & Kishi, K. High-Resolution Imaging of Lymphatic Vessels with Photoacoustic Lymphangiography. Radiology 292, 35 (2019).
Google Scholar
Ringstad, G. & Eide, P. K. Glymphatic-lymphatic coupling: assessment of the evidence from magnetic resonance imaging of humans. Cell Mol. Life Sci. 81, 131 (2024).
Google Scholar
Sun, B. et al. NIR-II nanoprobes for investigating the glymphatic system function under anesthesia and stroke injury. J. Nanobiotechnol. 22, 200 (2024).
Google Scholar
Yang, F. et al. Advancing insights into in vivo meningeal lymphatic vessels with stereoscopic wide-field photoacoustic microscopy. Light Sci. Appl 13, 96 (2024).
Google Scholar
Wu, C. H. et al. Noninvasive Characterization of Human Glymphatics and Meningeal Lymphatics in an in vivo Model of Blood-Brain Barrier Leakage. Ann. Neurol. 89, 111–124 (2021).
Google Scholar
Sennfalt, S. et al. Visualising and semi-quantitatively measuring brain fluid pathways, including meningeal lymphatics, in humans using widely available MRI techniques. J. Cereb. Blood Flow. Metab. 43, 1779–1795 (2023).
Google Scholar
Zhang, M. et al. Evaluation of glymphatic-meningeal lymphatic system with intravenous gadolinium-based contrast-enhancement in cerebral small-vessel disease. Eur. Radio. 33, 6096–6106 (2023).
Google Scholar
Wu, Y. et al. Borneol-driven meningeal lymphatic drainage clears amyloid-beta peptide to attenuate Alzheimer-like phenotype in mice. Theranostics 13, 106–124 (2023).
Google Scholar
Albayram, M. S. et al. Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes. Nat. Commun. 13, 203 (2022).
Google Scholar
Choe, K. et al. Intravital three-photon microscopy allows visualization over the entire depth of mouse lymph nodes. Nat. Immunol. 23, 330–340 (2022).
Google Scholar
Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013).
Google Scholar
Louveau, A. et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 21, 1380–1391 (2018).
Google Scholar
Wang, F. et al. Light-sheet microscopy in the near-infrared II window. Nat. Methods 16, 545–552 (2019).
Google Scholar
Tian, R. et al. Albumin-chaperoned cyanine dye yields superbright NIR-II fluorophore with enhanced pharmacokinetics. Sci. Adv. 5, eaaw0672, (2019).
Li, C. et al. Advanced Fluorescence Imaging Technology in the Near-Infrared-II Window for Biomedical Applications. J. Am. Chem. Soc. 142, 14789–14804 (2020).
Google Scholar
Cardinell, K. et al. A novel photoacoustic-fluorescent contrast agent for quantitative imaging of lymphatic drainage. Photoacoustics 21, 100239 (2021).
Google Scholar
Li, W. et al. Near-Infrared-II Imaging Revealed Hypothermia Regulates Neuroinflammation Following Brain Injury by Increasing the Glymphatic Influx. ACS Nano 18, 13836–13848 (2024).
Google Scholar
Bechet, N. B. et al. Light sheet fluorescence microscopy of optically cleared brains for studying the glymphatic system. J. Cereb. Blood Flow. Metab. 40, 1975–1986 (2020).
Google Scholar
Keil, S. A. et al. Dynamic infrared imaging of cerebrospinal fluid tracer influx into the brain. Neurophotonics 9, 031915 (2022).
Google Scholar
Ma, Q. et al. Rapid lymphatic efflux limits cerebrospinal fluid flow to the brain. Acta Neuropathol. 137, 151–165 (2019).
Google Scholar
Gu, X. et al. Clearance of two organic nanoparticles from the brain via the paravascular pathway. J. Control Rel. 322, 31–41 (2020).
Google Scholar
Miyakoshi, L. M. et al. The state of brain activity modulates cerebrospinal fluid transport. Prog. Neurobiol. 229, 102512 (2023).
Google Scholar
Kajita, H. et al. Visualization of Lymphatic Vessels Using Photoacoustic Imaging. Keio J. Med. 70, 82–92 (2021).
Google Scholar
Watanabe, S. et al. Photoacoustic lymphangiography is a possible alternative for lymphedema staging. J. Vasc. Surg. Venous Lymphat Disord. 10, e1312 (2022).
Suzuki, Y. et al. Subcutaneous Lymphatic Vessels in the Lower Extremities: Comparison between Photoacoustic Lymphangiography and Near-Infrared Fluorescence Lymphangiography. Radiology 295, 469–474 (2020).
Google Scholar
Lillis, A. P. & Krishnamurthy, R. Photoacoustic Imaging Addresses a Long-standing Challenge in Lymphedema. Radiology 295, 475–477 (2020).
Google Scholar
Suzuki, Y. et al. Measurement of lymphatic vessel depth using photoacoustic imaging. Lasers Surg. Med. 55, 164–168 (2023).
Google Scholar
van Heumen, S. et al. Imaging of the Lymphatic Vessels for Surgical Planning: A Systematic Review. Ann. Surg. Oncol. 30, 462–479 (2023).
Google Scholar
Wang, Z. et al. Monitoring the perivascular cerebrospinal fluid dynamics of the glymphatic pathway using co-localized photoacoustic microscopy. Opt. Lett. 48, 2265–2268 (2023).
Google Scholar
He, X. Z. et al. High-resolution 3D demonstration of regional heterogeneity in the glymphatic system. J. Cereb. Blood Flow. Metab. 42, 2017–2031 (2022).
Google Scholar
Erturk, A. et al. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat. Protoc. 7, 1983–1995 (2012).
Google Scholar
Xie, Q. et al. Rewiring the Brain: The Next Frontier in Supermicrosurgery. Plast. Reconstr. Surg. 153, 494e–495e (2024).
Google Scholar
Wu, R. et al. Safety and efficacy of intracranial vascularized submental lymph node transfer for treating hydrocephalus. J. Surg. Oncol. 129, 26–31 (2024).
Google Scholar
Rustenhoven, J. et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell 184, e1027 (2021).
Google Scholar
Yang, J. F. et al. Understanding lymphangiogenesis in knockout models, the cornea, and ocular diseases for the development of therapeutic interventions. Surv. Ophthalmol. 61, 272–296 (2016).
Google Scholar
Matsushita, J. et al. Fluorescence and Bioluminescence Imaging of Angiogenesis in Flk1-Nano-lantern Transgenic Mice. Sci. Rep. 7, 46597 (2017).
Google Scholar
Kang, G. J. et al. Intravital Imaging Reveals Dynamics of Lymphangiogenesis and Valvulogenesis. Sci. Rep. 6, 19459 (2016).
Google Scholar
Calvo, C. F. et al. Vascular endothelial growth factor receptor 3 directly regulates murine neurogenesis. Genes Dev. 25, 831–844 (2011).
Google Scholar
Zhu, J. et al. Simultaneous in vivo imaging of blood and lymphatic vessel growth in Prox1-GFP/Flk1::myr-mCherry mice. FEBS J. 282, 1458–1467 (2015).
Google Scholar
Okabe, K. et al. Neurons limit angiogenesis by titrating VEGF in retina. Cell 159, 584–596 (2014).
Google Scholar
Zhong, W. et al. Prox1-GFP/Flt1-DsRed transgenic mice: an animal model for simultaneous live imaging of angiogenesis and lymphangiogenesis. Angiogenesis 20, 581–598 (2017).
Google Scholar
Oliver, G., Kipnis, J., Randolph, G. J. & Harvey, N. L. The Lymphatic Vasculature in the 21(st) Century: Novel Functional Roles in Homeostasis and Disease. Cell 182, 270–296 (2020).
Google Scholar
Bradbury, M. W. & Cole, D. F. The role of the lymphatic system in drainage of cerebrospinal fluid and aqueous humour. J. Physiol. 299, 353–365 (1980).
Google Scholar
Knopf, P. M. et al. Physiology and immunology of lymphatic drainage of interstitial and cerebrospinal fluid from the brain. Neuropathol. Appl Neurobiol. 21, 175–180 (1995).
Google Scholar
Jacob, L. et al. Anatomy and function of the vertebral column lymphatic network in mice. Nat. Commun. 10, 4594 (2019).
Google Scholar
Wang, L. et al. Deep cervical lymph node ligation aggravates AD-like pathology of APP/PS1 mice. Brain Pathol. 29, 176–192 (2019).
Google Scholar
Da Mesquita, S. et al. Meningeal lymphatics affect microglia responses and anti-Abeta immunotherapy. Nature 593, 255–260 (2021).
Google Scholar
Chen, X. et al. Cerebral amyloid angiopathy is associated with glymphatic transport reduction and time-delayed solute drainage along the neck arteries. Nat. Aging 2, 214–223 (2022).
Google Scholar
Cao, X. et al. Deletion of aquaporin-4 aggravates brain pathology after blocking of the meningeal lymphatic drainage. Brain Res. Bull. 143, 83–96 (2018).
Google Scholar
Pu, T. et al. Persistent Malfunction of Glymphatic and Meningeal Lymphatic Drainage in a Mouse Model of Subarachnoid Hemorrhage. Exp. Neurobiol. 28, 104–118 (2019).
Google Scholar
Zou, W. et al. Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated alpha-synuclein. Transl. Neurodegener. 8, 7 (2019).
Google Scholar
Oehmichen, M., Gruninger, H., Wietholter, H. & Gencic, M. Lymphatic efflux of intracerebrally injected cells. Acta Neuropathol. 45, 61–65 (1979).
Google Scholar
Oehmichen, M., Wietholter, H., Gruninger, H. & Gencic, M. Destruction of intracerebrally applied red blood cells in cervical lymph nodes. Experimental investigations. Forensic. Sci. Int. 21, 43–57 (1983).
Google Scholar
Chen, J. et al. Meningeal lymphatics clear erythrocytes that arise from subarachnoid hemorrhage. Nat. Commun. 11, 3159 (2020).
Google Scholar
Duan, M. et al. Targeting brain-peripheral immune responses for secondary brain injury after ischemic and hemorrhagic stroke. J. Neuroinflammation 21, 102 (2024).
Google Scholar
Kipnis, J. Multifaceted interactions between adaptive immunity and the central nervous system. Science 353, 766–771 (2016).
Google Scholar
Louveau, A., Harris, T. H. & Kipnis, J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 36, 569–577 (2015).
Google Scholar
Mokbel, A. Y., Burns, M. P. & Main, B. S. The contribution of the meningeal immune interface to neuroinflammation in traumatic brain injury. J. Neuroinflammation 21, 135 (2024).
Google Scholar
Shu, K. et al. Altered Brain Glymphatic Function at Diffusion-Tensor MRI in Pre-cirrhotic Metabolic Dysfunction-Associated Fatty Liver Disease. Acad. Radio. 31, 4946–4954 (2024).
Google Scholar
Frederick, N. & Louveau, A. Meningeal lymphatics, immunity and neuroinflammation. Curr. Opin. Neurobiol. 62, 41–47 (2020).
Google Scholar
Olate-Briones, A. et al. The meningeal lymphatic vasculature in neuroinflammation. FASEB J. 36, e22276 (2022).
Google Scholar
Tavares, G. A. & Louveau, A. Meningeal Lymphatics: An Immune Gateway for the Central Nervous System. Cells. 10, 3385 (2021).
Laaker, C. et al. Immune cells as messengers from the CNS to the periphery: the role of the meningeal lymphatic system in immune cell migration from the CNS. Front Immunol. 14, 1233908 (2023).
Google Scholar
Mestre, H., Mori, Y. & Nedergaard, M. The Brain’s Glymphatic System: Current Controversies. Trends Neurosci. 43, 458–466 (2020).
Google Scholar
Planas, A. M. et al. Brain-derived antigens in lymphoid tissue of patients with acute stroke. J. Immunol. 188, 2156–2163 (2012).
Google Scholar
Tsuchida, T. et al. Autoreactive CD8 + T-cell responses to human myelin protein-derived peptides. Proc. Natl Acad. Sci. USA 91, 10859–10863 (1994).
Google Scholar
Rojas, O. L. et al. Recirculating Intestinal IgA-Producing Cells Regulate Neuroinflammation via IL-10. Cell 177, 492–493 (2019).
Google Scholar
Brioschi, S. et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science. 373, eabf9277 (2021).
Rua, R. & McGavern, D. B. Advances in Meningeal Immunity. Trends Mol. Med 24, 542–559 (2018).
Google Scholar
Da Mesquita, S. et al. Aging-associated deficit in CCR7 is linked to worsened glymphatic function, cognition, neuroinflammation, and beta-amyloid pathology. Sci Adv. 7, eabe4601 (2021).
Rustenhoven, J. et al. Age-related alterations in meningeal immunity drive impaired CNS lymphatic drainage. J. Exp. Med. 220, e20221929 (2023).
Reines, I. et al. Topical application of sphingosine-1-phosphate and FTY720 attenuate allergic contact dermatitis reaction through inhibition of dendritic cell migration. J. Invest Dermatol. 129, 1954–1962 (2009).
Google Scholar
Cugurra, A. et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science. 373, eabf7844 (2021).
Drieu, A. et al. Parenchymal border macrophages regulate the flow dynamics of the cerebrospinal fluid. Nature 611, 585–593 (2022).
Google Scholar
Chang, J. et al. Characteristic Features of Deep Brain Lymphatic Vessels and Their Regulation by Chronic Stress. Research 6, 0120 (2023).
Google Scholar
Chen, J. et al. Cerebrovascular Injuries Induce Lymphatic Invasion into Brain Parenchyma to Guide Vascular Regeneration in Zebrafish. Dev. Cell 49, e695 (2019).
Google Scholar
Siret, C. et al. Deciphering the heterogeneity of the Lyve1(+) perivascular macrophages in the mouse brain. Nat. Commun. 13, 7366 (2022).
Google Scholar
An, C. et al. Molecular dialogs between the ischemic brain and the peripheral immune system: dualistic roles in injury and repair. Prog. Neurobiol. 115, 6–24 (2014).
Google Scholar
Kim, J. B., Lim, C. M., Yu, Y. M. & Lee, J. K. Induction and subcellular localization of high-mobility group box-1 (HMGB1) in the postischemic rat brain. J. Neurosci. Res. 86, 1125–1131 (2008).
Google Scholar
Bianchi, R., Kastrisianaki, E., Giambanco, I. & Donato, R. S100B protein stimulates microglia migration via RAGE-dependent up-regulation of chemokine expression and release. J. Biol. Chem. 286, 7214–7226 (2011).
Google Scholar
Zhang, M. et al. ATP induces mild hypothermia in rats but has a strikingly detrimental impact on focal cerebral ischemia. J. Cereb. Blood Flow. Metab. 33, e1–e10 (2013).
Google Scholar
Bourhy, L. et al. Neuro-Inflammatory Response and Brain-Peripheral Crosstalk in Sepsis and Stroke. Front Immunol. 13, 834649 (2022).
Google Scholar
Monsour, M. & Borlongan, C. V. The central role of peripheral inflammation in ischemic stroke. J. Cereb. Blood Flow. Metab. 43, 622–641 (2023).
Google Scholar
Wang, H. et al. Neuroinflammation and peripheral immunity: Focus on ischemic stroke. Int. Immunopharmacol. 120, 110332 (2023).
Google Scholar
Yu, H. et al. The “Dialogue” Between Central and Peripheral Immunity After Ischemic Stroke: Focus on Spleen. Front. Immunol. 12, 792522 (2021).
Google Scholar
Jian, Z. et al. The Involvement and Therapy Target of Immune Cells After Ischemic Stroke. Front. Immunol. 10, 2167 (2019).
Google Scholar
Cheng, W., Zhao, Q., Li, C. & Xu, Y. Neuroinflammation and brain-peripheral interaction in ischemic stroke: A narrative review. Front. Immunol. 13, 1080737 (2022).
Google Scholar
Xie, L., He, M., Ying, C. & Chu, H. Mechanisms of inflammation after ischemic stroke in brain-peripheral crosstalk. Front. Mol. Neurosci. 17, 1400808 (2024).
Google Scholar
Lee, G. A. et al. CCN1 Is a Therapeutic Target for Reperfused Ischemic Brain Injury. Transl. Stroke Res. (2024).
Wu, F. et al. Systemic immune responses after ischemic stroke: From the center to the periphery. Front. Immunol. 13, 911661 (2022).
Google Scholar
Esposito, E. et al. Brain-to-cervical lymph node signaling after stroke. Nat. Commun. 10, 5306 (2019).
Google Scholar
Zhu, X. et al. Surgery induces neurocognitive disorder via neuroinflammation and glymphatic dysfunction in middle-aged mice with brain lymphatic drainage impairment. Front. Neurosci. 18, 1426718 (2024).
Google Scholar
Hsu, M. et al. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat. Commun. 10, 229 (2019).
Google Scholar
Hsu, M. et al. Neuroinflammation creates an immune regulatory niche at the meningeal lymphatic vasculature near the cribriform plate. Nat. Immunol. 23, 581–593 (2022).
Google Scholar
Spera, I. et al. Open pathways for cerebrospinal fluid outflow at the cribriform plate along the olfactory nerves. EBioMedicine 91, 104558 (2023).
Google Scholar
Fitzpatrick, Z. et al. Venous-plexus-associated lymphoid hubs support meningeal humoral immunity. Nature 628, 612–619 (2024).
Google Scholar
Kirthivasan, N. & Cyster, J. G. Lymphoid tissue on the mind. Trends Immunol. 45, 325–326 (2024).
Google Scholar
Semyachkina-Glushkovskaya, O. et al. Intranasal Delivery of Liposomes to Glioblastoma by Photostimulation of the Lymphatic System. Pharmaceutics. 15, 36 (2022).
Makinen, T. Lymphatic vessels at the base of the mouse brain provide direct drainage to the periphery. Nature 572, 34–35 (2019).
Google Scholar
Maisel, K., Sasso, M. S., Potin, L. & Swartz, M. A. Exploiting lymphatic vessels for immunomodulation: Rationale, opportunities, and challenges. Adv. Drug Deliv. Rev. 114, 43–59 (2017).
Google Scholar
Qi, Y. et al. New trends in brain tumor immunity with the opportunities of lymph nodes targeted drug delivery. J. Nanobiotechnol. 21, 254 (2023).
Google Scholar
Zhao, P., Le, Z., Liu, L. & Chen, Y. Therapeutic Delivery to the Brain via the Lymphatic Vasculature. Nano Lett. 20, 5415–5420 (2020).
Google Scholar
Liau, L. M. et al. Association of Autologous Tumor Lysate-Loaded Dendritic Cell Vaccination With Extension of Survival Among Patients With Newly Diagnosed and Recurrent Glioblastoma: A Phase 3 Prospective Externally Controlled Cohort Trial. JAMA Oncol. 9, 112–121 (2023).
Google Scholar
Irschick, R., Siemon, C. & Brenner, E. The history of anatomical research of lymphatics – From the ancient times to the end of the European Renaissance. Ann. Anat. 223, 49–69 (2019).
Google Scholar
Breslin, J. W. et al. Lymphatic Vessel Network Structure and Physiology. Compr. Physiol. 9, 207–299 (2018).
Google Scholar
Hu, Z. et al. Lymphatic vessel: origin, heterogeneity, biological functions, and therapeutic targets. Signal Transduct. Target Ther. 9, 9 (2024).
Google Scholar
Natale, G., Bocci, G. & Ribatti, D. Scholars and scientists in the history of the lymphatic system. J. Anat. 231, 417–429 (2017).
Google Scholar
Makinen, T., Norrmen, C. & Petrova, T. V. Molecular mechanisms of lymphatic vascular development. Cell Mol. Life Sci. 64, 1915–1929 (2007).
Google Scholar
Simeroth, S. & Yu, P. The role of lymphatic endothelial cell metabolism in lymphangiogenesis and disease. Front. Cardiovasc. Med. 11, 1392816 (2024).
Google Scholar
Montenegro-Navarro, N., Garcia-Baez, C. & Garcia-Caballero, M. Molecular and metabolic orchestration of the lymphatic vasculature in physiology and pathology. Nat. Commun. 14, 8389 (2023).
Google Scholar
Deng, H. et al. Current Status of Lymphangiogenesis: Molecular Mechanism, Immune Tolerance, and Application Prospect. Cancers. 15, 1169 (2023).
Da Mesquita, S., Fu, Z. & Kipnis, J. The Meningeal Lymphatic System: A New Player in Neurophysiology. Neuron 100, 375–388 (2018).
Google Scholar
Joukov, V. et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 15, 1751 (1996).
Google Scholar
Jeltsch, M. et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 276, 1423–1425 (1997).
Google Scholar
Nurmi, H. et al. VEGF-C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Mol. Med. 7, 1418–1425 (2015).
Google Scholar
Karkkainen, M. J. et al. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat. Genet. 25, 153–159 (2000).
Google Scholar
Hogan, B. M. et al. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat. Genet. 41, 396–398 (2009).
Google Scholar
Bos, F. L. et al. CCBE1 is essential for mammalian lymphatic vascular development and enhances the lymphangiogenic effect of vascular endothelial growth factor-C in vivo. Circ. Res. 109, 486–491 (2011).
Google Scholar
Zou, Z. et al. The secreted lymphangiogenic factor CCBE1 is essential for fetal liver erythropoiesis. Blood 121, 3228–3236 (2013).
Google Scholar
Hagerling, R. et al. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J. 32, 629–644 (2013).
Google Scholar
Jeltsch, M. et al. CCBE1 enhances lymphangiogenesis via A disintegrin and metalloprotease with thrombospondin motifs-3-mediated vascular endothelial growth factor-C activation. Circulation 129, 1962–1971 (2014).
Google Scholar
Le Guen, L. et al. Ccbe1 regulates Vegfc-mediated induction of Vegfr3 signaling during embryonic lymphangiogenesis. Development 141, 1239–1249 (2014).
Google Scholar
Bui, H. M. et al. Proteolytic activation defines distinct lymphangiogenic mechanisms for VEGFC and VEGFD. J. Clin. Invest. 126, 2167–2180 (2016).
Google Scholar
Karkkainen, M. J. et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 5, 74–80 (2004).
Google Scholar
Adams, R. H. & Alitalo, K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 8, 464–478 (2007).
Google Scholar
Kajiya, K., Hirakawa, S. & Detmar, M. Vascular endothelial growth factor-A mediates ultraviolet B-induced impairment of lymphatic vessel function. Am. J. Pathol. 169, 1496–1503 (2006).
Google Scholar
Kuchler, A. M. et al. Development of the zebrafish lymphatic system requires VEGFC signaling. Curr. Biol. 16, 1244–1248 (2006).
Google Scholar
Wang, G. et al. Specific fibroblast subpopulations and neuronal structures provide local sources of Vegfc-processing components during zebrafish lymphangiogenesis. Nat. Commun. 11, 2724 (2020).
Google Scholar
Ocskay, Z. et al. CCBE1 regulates the development and prevents the age-dependent regression of meningeal lymphatics. Biomed. Pharmacother. 170, 116032 (2024).
Google Scholar
Boisserand, L. S. B. et al. VEGF-C prophylaxis favors lymphatic drainage and modulates neuroinflammation in a stroke model. J Exp Med. 221, e20221983 (2024).
Merlini, A. et al. Distinct roles of the meningeal layers in CNS autoimmunity. Nat. Neurosci. 25, 887–899 (2022).
Google Scholar
Kataru, R. P. et al. Lymphatic-specific intracellular modulation of receptor tyrosine kinase signaling improves lymphatic growth and function. Sci Signal. 14, eabc0836 (2021).
Alitalo, K. The lymphatic vasculature in disease. Nat. Med. 17, 1371–1380 (2011).
Google Scholar
Escobedo, N. & Oliver, G. Lymphangiogenesis: Origin, Specification, and Cell Fate Determination. Annu. Rev. Cell Dev. Biol. 32, 677–691 (2016).
Google Scholar
Thomas, S. N., Rohner, N. A. & Edwards, E. E. Implications of Lymphatic Transport to Lymph Nodes in Immunity and Immunotherapy. Annu. Rev. Biomed. Eng. 18, 207–233 (2016).
Google Scholar
Ujiie, N. & Kume, T. Mechanical forces in lymphatic vessel development: Focus on transcriptional regulation. Front. Physiol. 13, 1066460 (2022).
Google Scholar
Sabine, A. et al. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev. Cell 22, 430–445 (2012).
Google Scholar
Hernandez Vasquez, M. N. et al. Transcription factor FOXP2 is a flow-induced regulator of collecting lymphatic vessels. EMBO J. 40, e107192 (2021).
Google Scholar
Liebl, J. et al. Cdk5 controls lymphatic vessel development and function by phosphorylation of Foxc2. Nat. Commun. 6, 7274 (2015).
Google Scholar
Kazenwadel, J. et al. GATA2 is required for lymphatic vessel valve development and maintenance. J. Clin. Invest 125, 2979–2994 (2015).
Google Scholar
Brice, G. et al. Analysis of the phenotypic abnormalities in lymphoedema-distichiasis syndrome in 74 patients with FOXC2 mutations or linkage to 16q24. J. Med. Genet. 39, 478–483 (2002).
Google Scholar
Dagenais, S. L. et al. Foxc2 is expressed in developing lymphatic vessels and other tissues associated with lymphedema-distichiasis syndrome. Gene Expr. Patterns 4, 611–619 (2004).
Google Scholar
Ye, T. et al. Borneol regulates meningeal lymphatic valve plasticity to clear Abeta aggregates in the prevention of AD-like symptoms. Phytomedicine 130, 155753 (2024).
Google Scholar
Scallan, J. P. et al. Foxo1 deletion promotes the growth of new lymphatic valves. J Clin Invest. 131, e142341 (2021).
Niimi, K., Nakae, J., Inagaki, S. & Furuyama, T. FOXO1 represses lymphatic valve formation and maintenance via PRDM1. Cell Rep. 37, 110048 (2021).
Google Scholar
Kume, T. Lymphatic vessel development: fluid flow and valve-forming cells. J. Clin. Invest 125, 2924–2926 (2015).
Google Scholar
Sweet, D. T. et al. Lymph flow regulates collecting lymphatic vessel maturation in vivo. J. Clin. Invest 125, 2995–3007 (2015).
Google Scholar
Bazigou, E. & Makinen, T. Flow control in our vessels: vascular valves make sure there is no way back. Cell Mol. Life Sci. 70, 1055–1066 (2013).
Google Scholar
Xiao, B. Levering Mechanically Activated Piezo Channels for Potential Pharmacological Intervention. Annu Rev. Pharm. Toxicol. 60, 195–218 (2020).
Google Scholar
Nonomura, K. et al. Mechanically activated ion channel PIEZO1 is required for lymphatic valve formation. Proc. Natl Acad. Sci. USA 115, 12817–12822 (2018).
Google Scholar
Choi, D. et al. Piezo1 incorporates mechanical force signals into the genetic program that governs lymphatic valve development and maintenance. JCI Insight. 4, e125068 (2019).
Choi, D. et al. Piezo1-Regulated Mechanotransduction Controls Flow-Activated Lymphatic Expansion. Circ. Res. 131, e2–e21 (2022).
Google Scholar
Choi, D. et al. Laminar flow downregulates Notch activity to promote lymphatic sprouting. J. Clin. Invest 127, 1225–1240 (2017).
Google Scholar
Martin-Almedina, S., Mansour, S. & Ostergaard, P. Human phenotypes caused by PIEZO1 mutations; one gene, two overlapping phenotypes? J. Physiol. 596, 985–992 (2018).
Google Scholar
Syeda, R. et al. Chemical activation of the mechanotransduction channel Piezo1. Elife. 4, e07369 (2015).
Jantti, H. et al. Microglial amyloid beta clearance is driven by PIEZO1 channels. J. Neuroinflammation 19, 147 (2022).
Google Scholar
Munk, A. S. et al. PDGF-B Is Required for Development of the Glymphatic System. Cell Rep. 26, e2953 (2019).
Google Scholar
Xiang, T. et al. Effects of increased intracranial pressure on cerebrospinal fluid influx, cerebral vascular hemodynamic indexes, and cerebrospinal fluid lymphatic efflux. J. Cereb. Blood Flow. Metab. 42, 2287–2302 (2022).
Google Scholar
Jukkola, J. et al. Blood pressure lowering enhances cerebrospinal fluid efflux to the systemic circulation primarily via the lymphatic vasculature. Fluids Barriers CNS 21, 12 (2024).
Google Scholar
Stevenson, T. J., Hitpass Romero, K. & Rustenhoven, J. Meningeal lymphatics stem cognitive decline in craniosynostosis. Cell Stem Cell 30, 1395–1397 (2023).
Google Scholar
Aspelund, A. & Alitalo, K. Yoda1 opens the lymphatic path for craniosynostosis therapy. J Clin Invest. 134, e176858 (2024).
Planas-Paz, L. et al. Mechanoinduction of lymph vessel expansion. EMBO J. 31, 788–804 (2012).
Google Scholar
Choi, D. et al. Piezo1 regulates meningeal lymphatic vessel drainage and alleviates excessive CSF accumulation. Nat. Neurosci. 27, 913–926 (2024).
Google Scholar
Miao, A. et al. Brain clearance is reduced during sleep and anesthesia. Nat. Neurosci. 27, 1046–1050 (2024).
Google Scholar
Fyfe, I. Brain clearance not increased during sleep. Nat. Rev. Neurol. 20, 379 (2024).
Google Scholar
Malkki, H. Alzheimer disease: Sleep alleviates AD-related neuropathological processes. Nat. Rev. Neurol. 9, 657 (2013).
Google Scholar
Kress, B. T. et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 76, 845–861 (2014).
Google Scholar
Ma, L. et al. Skull progenitor cell-driven meningeal lymphatic restoration improves neurocognitive functions in craniosynostosis. Cell Stem Cell 30, e1477 (2023).
Google Scholar
Chen, Z. et al. MAP kinases. Chem. Rev. 101, 2449–2476 (2001).
Google Scholar
Deng, Y., Atri, D., Eichmann, A. & Simons, M. Endothelial ERK signaling controls lymphatic fate specification. J. Clin. Invest 123, 1202–1215 (2013).
Google Scholar
Gibot, L. et al. Cell-based approach for 3D reconstruction of lymphatic capillaries in vitro reveals distinct functions of HGF and VEGF-C in lymphangiogenesis. Biomaterials 78, 129–139 (2016).
Google Scholar
Sasaki, J. I. et al. VE-Cadherin and Anastomosis of Blood Vessels Formed by Dental Stem Cells. J. Dent. Res. 99, 437–445 (2020).
Google Scholar
Yamaguchi, K., Sudo, H. & Imai, K. Vascular endothelial growth factor signaling in VE-cadherin expression and tube-like formation by rheumatoid arthritic synovial fibroblast-like cells. Biochem. Biophys. Res. Commun. 508, 405–409 (2019).
Google Scholar
Norden, P. R. & Kume, T. Molecular Mechanisms Controlling Lymphatic Endothelial Junction Integrity. Front. Cell Dev. Biol. 8, 627647 (2020).
Google Scholar
Nakashima, B. J. & Hong, Y. K. VE-Cadherin: A Critical Sticking Point for Lymphatic System Maintenance: Role of VE-Cadherin in Lymphatic Maintenance. Circ. Res. 130, 24–26 (2022).
Google Scholar
Zhang, F., Zarkada, G., Yi, S. & Eichmann, A. Lymphatic Endothelial Cell Junctions: Molecular Regulation in Physiology and Diseases. Front Physiol. 11, 509 (2020).
Google Scholar
Meng, Y. et al. Temporospatial inhibition of Erk signaling is required for lymphatic valve formation. Sig. Transduct. Target Ther. 8, 342 (2023).
Google Scholar
Shrestha, N. et al. delta-Catenin Increases the Stability of EGFR by Decreasing c-Cbl Interaction and Enhances EGFR/Erk1/2 Signaling in Prostate Cancer. Mol. Cells 41, 320–330 (2018).
Google Scholar
Okuda, K. S. et al. 3,4-Difluorobenzocurcumin Inhibits Vegfc-Vegfr3-Erk Signalling to Block Developmental Lymphangiogenesis in Zebrafish. Pharmaceuticals 14, 614 (2021).
Greco, R. et al. Antagonism of CGRP Receptor: Central and Peripheral Mechanisms and Mediators in an Animal Model of Chronic Migraine. Cells. 11, 3092 (2022).
Brain, S. D. et al. Calcitonin gene-related peptide is a potent vasodilator. Nature 313, 54–56 (1985).
Google Scholar
Pawlak, J. B., Wetzel-Strong, S. E., Dunn, M. K. & Caron, K. M. Cardiovascular effects of exogenous adrenomedullin and CGRP in Ramp and Calcrl deficient mice. Peptides 88, 1–7 (2017).
Google Scholar
Petrova, T. V. et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 21, 4593–4599 (2002).
Google Scholar
Hirakawa, S. et al. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am. J. Pathol. 162, 575–586 (2003).
Google Scholar
Mackie, D. I. et al. hCALCRL mutation causes autosomal recessive nonimmune hydrops fetalis with lymphatic dysplasia. J. Exp. Med. 215, 2339–2353 (2018).
Google Scholar
Dackor, R. T. et al. Hydrops fetalis, cardiovascular defects, and embryonic lethality in mice lacking the calcitonin receptor-like receptor gene. Mol. Cell Biol. 26, 2511–2518 (2006).
Google Scholar
Davis, R. B. et al. Lymphatic deletion of calcitonin receptor-like receptor exacerbates intestinal inflammation. JCI Insight 2, e92465 (2017).
Google Scholar
Davis, R. B. et al. Calcitonin-Receptor-Like Receptor Signaling Governs Intestinal Lymphatic Innervation and Lipid Uptake. ACS Pharm. Transl. Sci. 2, 114–121 (2019).
Google Scholar
McLatchie, L. M. et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393, 333–339 (1998).
Google Scholar
Kamitani, S. et al. The RAMP2/CRLR complex is a functional adrenomedullin receptor in human endothelial and vascular smooth muscle cells. FEBS Lett. 448, 111–114 (1999).
Google Scholar
Hay, D. L., Poyner, D. R. & Sexton, P. M. GPCR modulation by RAMPs. Pharm. Ther. 109, 173–197 (2006).
Google Scholar
Woolley, M. J. & Conner, A. C. Comparing the molecular pharmacology of CGRP and adrenomedullin. Curr. Protein Pept. Sci. 14, 358–374 (2013).
Google Scholar
Mishima, T. et al. RAMP1 signaling improves lymphedema and promotes lymphangiogenesis in mice. J. Surg. Res. 219, 50–60 (2017).
Google Scholar
Johnson, K. W., Morin, S. M., Wroblewski, V. J. & Johnson, M. P. Peripheral and central nervous system distribution of the CGRP neutralizing antibody [(125)I] galcanezumab in male rats. Cephalalgia 39, 1241–1248 (2019).
Google Scholar
Mikhailov, N. et al. The role of the meningeal lymphatic system in local meningeal inflammation and trigeminal nociception. Sci. Rep. 12, 8804 (2022).
Google Scholar
Nelson-Maney, N. P. et al. Meningeal lymphatic CGRP signaling governs pain via cerebrospinal fluid efflux and neuroinflammation in migraine models. J. Clin. Invest. 134, e175616 (2024).
Fritz-Six, K. L., Dunworth, W. P., Li, M. & Caron, K. M. Adrenomedullin signaling is necessary for murine lymphatic vascular development. J. Clin. Invest 118, 40–50 (2008).
Google Scholar
Huisa, B. N. et al. Transcranial laser therapy for acute ischemic stroke: a pooled analysis of NEST-1 and NEST-2. Int. J. Stroke 8, 315–320 (2013).
Google Scholar
Hamblin, M. R. Photobiomodulation for traumatic brain injury and stroke. J. Neurosci. Res. 96, 731–743 (2018).
Google Scholar
Morries, L. D., Cassano, P. & Henderson, T. A. Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr. Dis. Treat. 11, 2159–2175 (2015).
Google Scholar
Semyachkina-Glushkovskaya, O. et al. Photobiomodulation of lymphatic drainage and clearance: perspective strategy for augmentation of meningeal lymphatic functions. Biomed. Opt. Express 11, 725–734 (2020).
Google Scholar
Dirican, A. et al. The short-term effects of low-level laser therapy in the management of breast-cancer-related lymphedema. Support Care Cancer 19, 685–690 (2011).
Google Scholar
Karu, T. I., Pyatibrat, L. V. & Afanasyeva, N. I. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg. Med 36, 307–314 (2005).
Google Scholar
Cassano, P. et al. Review of transcranial photobiomodulation for major depressive disorder: targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics 3, 031404 (2016).
Google Scholar
Xuan, W. et al. Low-level laser therapy for traumatic brain injury in mice increases brain derived neurotrophic factor (BDNF) and synaptogenesis. J. Biophotonics 8, 502–511 (2015).
Google Scholar
Tian, F., Hase, S. N., Gonzalez-Lima, F. & Liu, H. Transcranial laser stimulation improves human cerebral oxygenation. Lasers Surg. Med. 48, 343–349 (2016).
Google Scholar
Zinchenko, E. et al. Pilot study of transcranial photobiomodulation of lymphatic clearance of beta-amyloid from the mouse brain: breakthrough strategies for non-pharmacologic therapy of Alzheimer’s disease. Biomed. Opt. Express 10, 4003–4017 (2019).
Google Scholar
Wang, M. et al. Non-invasive modulation of meningeal lymphatics ameliorates ageing and Alzheimer’s disease-associated pathology and cognition in mice. Nat. Commun. 15, 1453 (2024).
Google Scholar
Salehpour, F., Khademi, M., Bragin, D. E. & DiDuro, J. O. Photobiomodulation Therapy and the Glymphatic System: Promising Applications for Augmenting the Brain Lymphatic Drainage System. Int. J. Mol. Sci. 23, 2975 (2022).
Broman, M. T., Mehta, D. & Malik, A. B. Cdc42 regulates the restoration of endothelial adherens junctions and permeability. Trends Cardiovasc Med 17, 151–156 (2007).
Google Scholar
de Freitas, L. F. & Hamblin, M. R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 22, 7000417 (2016).
Waypa, G. B., Smith, K. A. & Schumacker, P. T. O2 sensing, mitochondria and ROS signaling: The fog is lifting. Mol. Asp. Med 47-48, 76–89 (2016).
Google Scholar
Belenichev, I. et al. Modulating Nitric Oxide: Implications for Cytotoxicity and Cytoprotection. Antioxidants. 13, 504 (2024).
Drapier, J. C. et al. Biosynthesis of nitric oxide activates iron regulatory factor in macrophages. EMBO J. 12, 3643–3649 (1993).
Google Scholar
Ohhashi, T. et al. Physiological Roles of Lymph Flow-Mediated Nitric Oxide in Lymphatic System. Lymphat Res. Biol. 21, 253–261 (2023).
Google Scholar
Zhao, Y., Vanhoutte, P. M. & Leung, S. W. Vascular nitric oxide: Beyond eNOS. J. Pharm. Sci. 129, 83–94 (2015).
Google Scholar
Lepoivre, M. et al. Inactivation of ribonucleotide reductase by nitric oxide. Biochem. Biophys. Res Commun. 179, 442–448 (1991).
Google Scholar
Hagendoorn, J., Padera, T. P., Fukumura, D. & Jain, R. K. Molecular regulation of microlymphatic formation and function: role of nitric oxide. Trends Cardiovasc. Med. 15, 169–173 (2005).
Google Scholar
Isbell, T. S., Gladwin, M. T. & Patel, R. P. Hemoglobin oxygen fractional saturation regulates nitrite-dependent vasodilation of aortic ring bioassays. Am. J. Physiol. Heart Circ. Physiol. 293, H2565–H2572 (2007).
Google Scholar
Cosby, K. et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 9, 1498–1505 (2003).
Google Scholar
Helms, C. & Kim-Shapiro, D. B. Hemoglobin-mediated nitric oxide signaling. Free Radic. Biol. Med. 61, 464–472 (2013).
Google Scholar
Crawford, J. H. et al. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood 107, 566–574 (2006).
Google Scholar
Murad, F. Discovery of some of the biological effects of nitric oxide and its role in cell signaling. Biosci. Rep. 24, 452–474 (2004).
Google Scholar
Bohlen, H. G. et al. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am. J. Physiol. Heart Circ. Physiol. 297, H1319–H1328 (2009).
Google Scholar
Imai, T. et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 91, 521–530 (1997).
Google Scholar
Landsman, L. et al. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood 113, 963–972 (2009).
Google Scholar
Harrison, J. K. et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc. Natl Acad. Sci. USA 95, 10896–10901 (1998).
Google Scholar
Lucas, A. D. et al. The transmembrane form of the CX3CL1 chemokine fractalkine is expressed predominantly by epithelial cells in vivo. Am. J. Pathol. 158, 855–866 (2001).
Google Scholar
Greaves, D. R. et al. Linked Chromosome 16q13 Chemokines, Macrophage-Derived Chemokine, Fractalkine, and Thymus- and Activation-Regulated Chemokine, Are Expressed in Human Atherosclerotic Lesions. Arteriosclerosis Thrombosis Vasc. Biol. 21, 923–929 (2001).
Google Scholar
Bazan, J. F. et al. A new class of membrane-bound chemokine with a CX3C motif. Nature 385, 640–644 (1997).
Google Scholar
Ahn, S. Y. et al. Tumor necrosis factor-alpha induces fractalkine expression preferentially in arterial endothelial cells and mithramycin A suppresses TNF-alpha-induced fractalkine expression. Am. J. Pathol. 164, 1663–1672 (2004).
Google Scholar
Wong, B. W., Wong, D. & McManus, B. M. Characterization of fractalkine (CX3CL1) and CX3CR1 in human coronary arteries with native atherosclerosis, diabetes mellitus, and transplant vascular disease. Cardiovasc. Pathol. 11, 332–338 (2002).
Google Scholar
Lucas, A. D. et al. Smooth muscle cells in human atherosclerotic plaques express the fractalkine receptor CX3CR1 and undergo chemotaxis to the CX3C chemokine fractalkine (CX3CL1). Circulation 108, 2498–2504 (2003).
Google Scholar
Yang, X. P. et al. Fractalkine upregulates intercellular adhesion molecule-1 in endothelial cells through CX3CR1 and the Jak Stat5 pathway. Circ. Res. 101, 1001–1008 (2007).
Google Scholar
Qian, S. et al. “Find-eat” strategy targeting endothelial cells via receptor functionalized apoptotic body nanovesicle. Sci. Bull. 68, 826–837 (2023).
Google Scholar
Takeda, A. et al. Single-Cell Survey of Human Lymphatics Unveils Marked Endothelial Cell Heterogeneity and Mechanisms of Homing for Neutrophils. Immunity 51, e565 (2019).
Google Scholar
Fujimoto, N. & Dieterich, L. C. Mechanisms and Clinical Significance of Tumor Lymphatic Invasion. Cells. 10, 2585 (2021).
Jalkanen, S. & Salmi, M. Lymphatic endothelial cells of the lymph node. Nat. Rev. Immunol. 20, 566–578 (2020).
Google Scholar
Petrova, T. V. & Koh, G. Y. Biological functions of lymphatic vessels. Science. 369, eaax4063 (2020).
das Neves, S. P. et al. Meningeal lymphatic function promotes oligodendrocyte survival and brain myelination. Immunity 57, e2328 (2024).
Google Scholar
Butler, M. G., Dagenais, S. L., Rockson, S. G. & Glover, T. W. A novel VEGFR3 mutation causes Milroy disease. Am. J. Med. Genet. A 143A, 1212–1217 (2007).
Google Scholar
Butler, M. G., Isogai, S. & Weinstein, B. M. Lymphatic development. Birth Defects Res. C. Embryo Today 87, 222–231 (2009).
Google Scholar
Ferrell, R. E. et al. Hereditary lymphedema: evidence for linkage and genetic heterogeneity. Hum. Mol. Genet. 7, 2073–2078 (1998).
Google Scholar
Irrthum, A. et al. Congenital hereditary lymphedema caused by a mutation that inactivates VEGFR3 tyrosine kinase. Am. J. Hum. Genet. 67, 295–301 (2000).
Google Scholar
Falls, H. F. & Kertesz, E. D. A New Syndrome Combining Pterygium Colli with Developmental Anomalies of the Eyelids and Lymphatics of the Lower Extremities. Trans. Am. Ophthalmol. Soc. 62, 248–275 (1964).
Google Scholar
Fang, J. et al. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am. J. Hum. Genet. 67, 1382–1388 (2000).
Google Scholar
Finegold, D. N. et al. Truncating mutations in FOXC2 cause multiple lymphedema syndromes. Hum. Mol. Genet. 10, 1185–1189 (2001).
Google Scholar
Ghalamkarpour, A. et al. Sporadic in utero generalized edema caused by mutations in the lymphangiogenic genes VEGFR3 and FOXC2. J. Pediatr. 155, 90–93 (2009).
Google Scholar
Petrova, T. V. et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat. Med. 10, 974–981 (2004).
Google Scholar
van Steensel, M. A. et al. Novel missense mutations in the FOXC2 gene alter transcriptional activity. Hum. Mutat. 30, E1002–E1009 (2009).
Google Scholar
Jha, S. K. et al. Efficient activation of the lymphangiogenic growth factor VEGF-C requires the C-terminal domain of VEGF-C and the N-terminal domain of CCBE1. Sci. Rep. 7, 4916 (2017).
Google Scholar
Alders, M. et al. Evaluation of Clinical Manifestations in Patients with Severe Lymphedema with and without CCBE1 Mutations. Mol. Syndromol. 4, 107–113 (2013).
Google Scholar
Connell, F. et al. Linkage and sequence analysis indicate that CCBE1 is mutated in recessively inherited generalised lymphatic dysplasia. Hum. Genet. 127, 231–241 (2010).
Google Scholar
Alders, M. et al. Mutations in CCBE1 cause generalized lymph vessel dysplasia in humans. Nat. Genet. 41, 1272–1274 (2009).
Google Scholar
Van Balkom, I. D. et al. Lymphedema-lymphangiectasia-mental retardation (Hennekam) syndrome: a review. Am. J. Med. Genet. 112, 412–421 (2002).
Google Scholar
Hennekam, R. C. et al. Autosomal recessive intestinal lymphangiectasia and lymphedema, with facial anomalies and mental retardation. Am. J. Med. Genet. 34, 593–600 (1989).
Google Scholar
Geng, X. et al. Multiple mouse models of primary lymphedema exhibit distinct defects in lymphovenous valve development. Dev. Biol. 409, 218–233 (2016).
Google Scholar
Hahn, C. N. et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat. Genet. 43, 1012–1017 (2011).
Google Scholar
Kazenwadel, J. et al. Loss-of-function germline GATA2 mutations in patients with MDS/AML or MonoMAC syndrome and primary lymphedema reveal a key role for GATA2 in the lymphatic vasculature. Blood 119, 1283–1291 (2012).
Google Scholar
Ostergaard, P. et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat. Genet. 43, 929–931 (2011).
Google Scholar
Fotiou, E. et al. Novel mutations in PIEZO1 cause an autosomal recessive generalized lymphatic dysplasia with non-immune hydrops fetalis. Nat. Commun. 6, 8085 (2015).
Google Scholar
Francois, M. et al. Sox18 induces development of the lymphatic vasculature in mice. Nature 456, 643–647 (2008).
Google Scholar
Irrthum, A. et al. Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia. Am. J. Hum. Genet. 72, 1470–1478 (2003).
Google Scholar
Pennisi, D. et al. Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nat. Genet. 24, 434–437 (2000).
Google Scholar
Alders, M. et al. Hennekam syndrome can be caused by FAT4 mutations and be allelic to Van Maldergem syndrome. Hum. Genet. 133, 1161–1167 (2014).
Google Scholar
Betterman, K. L. et al. Atypical cadherin FAT4 orchestrates lymphatic endothelial cell polarity in response to flow. J. Clin. Invest 130, 3315–3328 (2020).
Google Scholar
Pujol, F. et al. Dachsous1-Fat4 Signaling Controls Endothelial Cell Polarization During Lymphatic Valve Morphogenesis-Brief Report. Arterioscler Thromb. Vasc. Biol. 37, 1732–1735 (2017).
Google Scholar
Brouillard, P. et al. Loss of ADAMTS3 activity causes Hennekam lymphangiectasia-lymphedema syndrome 3. Hum. Mol. Genet. 26, 4095–4104 (2017).
Google Scholar
Boone, P. M. et al. Biallelic mutation of FBXL7 suggests a novel form of Hennekam syndrome. Am. J. Med. Genet. A 182, 189–194 (2020).
Google Scholar
Lyons, O. et al. Human venous valve disease caused by mutations in FOXC2 and GJC2. J. Exp. Med. 214, 2437–2452 (2017).
Google Scholar
Ostergaard, P. et al. Rapid identification of mutations in GJC2 in primary lymphoedema using whole exome sequencing combined with linkage analysis with delineation of the phenotype. J. Med Genet. 48, 251–255 (2011).
Google Scholar
Ferrell, R. E. et al. GJC2 missense mutations cause human lymphedema. Am. J. Hum. Genet. 86, 943–948 (2010).
Google Scholar
Au, A. C. et al. Protein tyrosine phosphatase PTPN14 is a regulator of lymphatic function and choanal development in humans. Am. J. Hum. Genet. 87, 436–444 (2010).
Google Scholar
Ostergaard, P. et al. Mutations in KIF11 cause autosomal-dominant microcephaly variably associated with congenital lymphedema and chorioretinopathy. Am. J. Hum. Genet. 90, 356–362 (2012).
Google Scholar
Bazigou, E. et al. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev. Cell 17, 175–186 (2009).
Google Scholar
Huang, X. Z. et al. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol. Cell Biol. 20, 5208–5215 (2000).
Google Scholar
Ma, G. C. et al. A recurrent ITGA9 missense mutation in human fetuses with severe chylothorax: possible correlation with poor response to fetal therapy. Prenat. Diagn. 28, 1057–1063 (2008).
Google Scholar
Hong, S. E. et al. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat. Genet. 26, 93–96 (2000).
Google Scholar
Lutter, S., Xie, S., Tatin, F. & Makinen, T. Smooth muscle-endothelial cell communication activates Reelin signaling and regulates lymphatic vessel formation. J. Cell Biol. 197, 837–849 (2012).
Google Scholar
Martin-Almedina, S. et al. EPHB4 kinase-inactivating mutations cause autosomal dominant lymphatic-related hydrops fetalis. J. Clin. Invest 126, 3080–3088 (2016).
Google Scholar
Guo, X. et al. Emerging Roles of Meningeal Lymphatic Vessels in Alzheimer’s Disease. J. Alzheimers Dis. 94, S355–S366 (2023).
Google Scholar
Li, G. et al. The meningeal lymphatic vessels and the glymphatic system: Potential therapeutic targets in neurological disorders. J. Cereb. Blood Flow. Metab. 42, 1364–1382 (2022).
Google Scholar
Wang, Y. & Oliver, G. Current views on the function of the lymphatic vasculature in health and disease. Genes Dev. 24, 2115–2126 (2010).
Google Scholar
Semyachkina-Glushkovskaya, O. et al. Application of optical coherence tomography for in vivo monitoring of the meningeal lymphatic vessels during opening of blood-brain barrier: mechanisms of brain clearing. J. Biomed. Opt. 22, 1–9 (2017).
Google Scholar
Iliff, J. J. et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 33, 18190–18199 (2013).
Google Scholar
Holstein-Ronsbo, S. et al. Glymphatic influx and clearance are accelerated by neurovascular coupling. Nat. Neurosci. 26, 1042–1053 (2023).
Google Scholar
Kilic, K. & Devor, A. The stop and go of glymphatic flow. Nat. Neurosci. 26, 924–925 (2023).
Google Scholar
Xiong, Y. et al. Advances in the study of the glymphatic system and aging. CNS Neurosci. Ther. 30, e14803 (2024).
Google Scholar
Li, W. et al. Modulation of lymphatic transport in the central nervous system. Theranostics 12, 1117–1131 (2022).
Google Scholar
Pla, V. et al. A real-time in vivo clearance assay for quantification of glymphatic efflux. Cell Rep. 40, 111320 (2022).
Google Scholar
Rego, S., Sanchez, G. & Da Mesquita, S. Current views on meningeal lymphatics and immunity in aging and Alzheimer’s disease. Mol. Neurodegener. 18, 55 (2023).
Google Scholar
Salvador, A. F. M., Abduljawad, N. & Kipnis, J. Meningeal Lymphatics in Central Nervous System Diseases. Annu Rev. Neurosci. 47, 323–344 (2024).
Google Scholar
Yamada, K. & Iwatsubo, T. Involvement of the glymphatic/meningeal lymphatic system in Alzheimer’s disease: insights into proteostasis and future directions. Cell Mol. Life Sci. 81, 192 (2024).
Google Scholar
Chen, J. et al. The lymphatic drainage system of the CNS plays a role in lymphatic drainage, immunity, and neuroinflammation in stroke. J. Leukoc. Biol. 110, 283–291 (2021).
Google Scholar
Hladky, S. B. & Barrand, M. A. The glymphatic hypothesis: the theory and the evidence. Fluids Barriers CNS 19, 9 (2022).
Google Scholar
Yankova, G., Bogomyakova, O. & Tulupov, A. The glymphatic system and meningeal lymphatics of the brain: new understanding of brain clearance. Rev. Neurosci. 32, 693–705 (2021).
Google Scholar
Chen, J. et al. The Interplay between Meningeal Lymphatic Vessels and Neuroinflammation in Neurodegenerative Diseases. Curr. Neuropharmacol. 22, 1016–1032 (2024).
Google Scholar
Abbaoui, A., Fatoba, O. & Yamashita, T. Meningeal T cells function in the central nervous system homeostasis and neurodegenerative diseases. Front Cell Neurosci. 17, 1181071 (2023).
Google Scholar
Ma, T., Wang, F., Xu, S. & Huang, J. H. Meningeal immunity: Structure, function and a potential therapeutic target of neurodegenerative diseases. Brain Behav. Immun. 93, 264–276 (2021).
Google Scholar
Jiang-Xie, L. F., Drieu, A. & Kipnis, J. Waste clearance shapes aging brain health. Neuron, 113, 71–81 (2025).
Proulx, S. T. & Engelhardt, B. Macrophages clear the way for CNS fluid flow. Lancet Neurol. 22, 194–195 (2023).
Google Scholar
Bordon, Y. Macrophages bordering the brain parenchyma regulate the flow of cerebrospinal fluid. Nat. Rev. Immunol. 23, 3 (2023).
Google Scholar
Da Mesquita, S. & Rua, R. Brain border-associated macrophages: common denominators in infection, aging, and Alzheimer’s disease? Trends Immunol. 45, 346–357 (2024).
Google Scholar
Van Hove, H. et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 22, 1021–1035 (2019).
Google Scholar
Drieu, A. et al. Parenchymal border macrophages regulate tau pathology and tau-mediated neurodegeneration. Life Sci Alliance. 6, (2023).
Corder, E. H. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923 (1993).
Google Scholar
Genin, E. et al. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol. Psychiatry 16, 903–907 (2011).
Google Scholar
Theendakara, V., Peters-Libeu, C. A., Bredesen, D. E. & Rao, R. V. Transcriptional Effects of ApoE4: Relevance to Alzheimer’s Disease. Mol. Neurobiol. 55, 5243–5254 (2018).
Google Scholar
Montagne, A. et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature 581, 71–76 (2020).
Google Scholar
Blanchard, J. W. et al. Reconstruction of the human blood-brain barrier in vitro reveals a pathogenic mechanism of APOE4 in pericytes. Nat. Med. 26, 952–963 (2020).
Google Scholar
Achariyar, T. M. et al. Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol. Neurodegener. 11, 74 (2016).
Google Scholar
Xu, Q. et al. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J. Neurosci. 26, 4985–4994 (2006).
Google Scholar
Mentis, A. A., Dardiotis, E. & Chrousos, G. P. Apolipoprotein E4 and meningeal lymphatics in Alzheimer disease: a conceptual framework. Mol. Psychiatry 26, 1075–1097 (2021).
Google Scholar
Chen, Z., Schwulst, S. J. & Mentis, A. A. APOE4-mediated Alzheimer disease and “Vascular”-“Meningeal Lymphatic” components: towards a novel therapeutic era? Mol. Psychiatry 26, 5472–5474 (2021).
Google Scholar
Konialis, C. et al. The APOE E4 Allele Confers Increased Risk of Ischemic Stroke Among Greek Carriers. Adv. Clin. Exp. Med. 25, 471–478 (2016).
Google Scholar
Zhou, Y. et al. Impaired Meningeal Lymphatics and Glymphatic Pathway in Patients with White Matter Hyperintensity. Adv. Sci. 11, e2402059 (2024).
Google Scholar
Huang, Z., Hamblin, M. R. & Zhang, Q. Photobiomodulation in experimental models of Alzheimer’s disease: state-of-the-art and translational perspectives. Alzheimers Res. Ther. 16, 114 (2024).
Google Scholar
Yang, L. et al. Non-invasive photobiomodulation treatment in an Alzheimer Disease-like transgenic rat model. Theranostics 12, 2205–2231 (2022).
Google Scholar
Shan, X. et al. A Long-Acting Lyotropic Liquid Crystalline Implant Promotes the Drainage of Macromolecules by Brain-Related Lymphatic System in Treating Aged Alzheimer’s Disease. ACS Nano 18, 9688–9703 (2024).
Google Scholar
Semyachkina-Glushkovskaya, O. et al. Night Photostimulation of Clearance of Beta-Amyloid from Mouse Brain: New Strategies in Preventing Alzheimer’s Disease. Cells. 10, 3289 (2021).
Wang, H. C. et al. BV2 Membrane-Coated PEGylated-Liposomes Delivered hFGF21 to Cortical and Hippocampal Microglia for Alzheimer’s Disease Therapy. Adv. Health. Mater. 13, e2400125 (2024).
Google Scholar
Poewe, W. et al. Parkinson disease. Nat. Rev. Dis. Prim. 3, 17013 (2017).
Google Scholar
Armstrong, M. J. & Okun, M. S. Diagnosis and Treatment of Parkinson Disease. JAMA 323, 548–560 (2020).
Google Scholar
Dorsey, E. R. et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68, 384–386 (2007).
Google Scholar
Kalia, L. V. & Lang, A. E. Parkinson’s disease. Lancet 386, 896–912 (2015).
Google Scholar
Charvin, D., Medori, R., Hauser, R. A. & Rascol, O. Therapeutic strategies for Parkinson disease: beyond dopaminergic drugs. Nat. Rev. Drug Discov. 17, 804–822 (2018).
Google Scholar
Margolesky, J. & Singer, C. Extended-release oral capsule of carbidopa-levodopa in Parkinson disease. Ther. Adv. Neurol. Disord. 11, 1756285617737728 (2018).
Google Scholar
Montastruc, J. L., Rascol, O. & Senard, J. M. Glutamate antagonists and Parkinson’s disease: a review of clinical data. Neurosci. Biobehav Rev. 21, 477–480 (1997).
Google Scholar
Sharma, G. et al. Advances in nanocarriers enabled brain targeted drug delivery across blood brain barrier. Int. J. Pharm. 559, 360–372 (2019).
Google Scholar
Zhang, G. et al. Thin platelet-like COF nanocomposites for blood brain barrier transport and inhibition of brain metastasis from renal cancer. J. Mater. Chem. B 8, 4475–4488 (2020).
Google Scholar
Mantri, S. et al. Patterns of Dementia Treatment and Frank Prescribing Errors in Older Adults With Parkinson Disease. JAMA Neurol. 76, 41–49 (2019).
Google Scholar
Wang, J. T. et al. Enhanced Delivery of Neuroactive Drugs via Nasal Delivery with a Self-Healing Supramolecular Gel. Adv. Sci. 8, e2101058 (2021).
Google Scholar
de Oliveira Junior, E. R. et al. Nasal administration of nanoencapsulated geraniol/ursodeoxycholic acid conjugate: Towards a new approach for the management of Parkinson’s disease. J. Control Rel. 321, 540–552 (2020).
Google Scholar
Ozsoy, Y., Gungor, S. & Cevher, E. Nasal delivery of high molecular weight drugs. Molecules 14, 3754–3779 (2009).
Google Scholar
Liu, J. et al. Delivery of Biomimetic Liposomes via Meningeal Lymphatic Vessels Route for Targeted Therapy of Parkinson’s Disease. Research 6, 0030 (2023).
Google Scholar
Ma, Z. et al. Traumatic brain injury in elderly population: A global systematic review and meta-analysis of in-hospital mortality and risk factors among 2.22 million individuals. Ageing Res. Rev. 99, 102376 (2024).
Google Scholar
van Hameren, G. et al. From spreading depolarization to blood-brain barrier dysfunction: navigating traumatic brain injury for novel diagnosis and therapy. Nat. Rev. Neurol. 20, 408–425 (2024).
Google Scholar
Cash, A. & Theus, M. H. Mechanisms of Blood-Brain Barrier Dysfunction in Traumatic Brain Injury. Int. J. Mol. Sci. 21, 3344 (2020).
Overgaard Wichmann, T., Hedegaard Hojsager, M. & Hasager Damkier, H. Water channels in the brain and spinal cord-overview of the role of aquaporins in traumatic brain injury and traumatic spinal cord injury. Front. Cell Neurosci. 18, 1414662 (2024).
Google Scholar
Shang, P. et al. New Insights on Mechanisms and Therapeutic Targets of Cerebral Edema. Curr. Neuropharmacol. 22, 2330–2352 (2024).
Google Scholar
Lempriere, S. Meningeal lymphatic flow slows after mild traumatic brain injury. Nat. Rev. Neurol. 16, 600 (2020).
Google Scholar
Puy, L. et al. Intracerebral haemorrhage. Nat. Rev. Dis. Prim. 9, 14 (2023).
Google Scholar
Collaborators, G. B. D. S. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 20, 795–820 (2021).
Google Scholar
Poon, M. T., Fonville, A. F. & Al-Shahi Salman, R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 85, 660–667 (2014).
Google Scholar
Moulin, S. et al. Dementia risk after spontaneous intracerebral haemorrhage: a prospective cohort study. Lancet Neurol. 15, 820–829 (2016).
Google Scholar
Li, L. et al. Risks of recurrent stroke and all serious vascular events after spontaneous intracerebral haemorrhage: pooled analyses of two population-based studies. Lancet Neurol. 20, 437–447 (2021).
Google Scholar
Zhang, X. et al. Iron/ROS/Itga3 mediated accelerated depletion of hippocampal neural stem cell pool contributes to cognitive impairment after hemorrhagic stroke. Redox Biol. 71, 103086 (2024).
Google Scholar
Shah, V. A. et al. One-Year Outcome Trajectories and Factors Associated with Functional Recovery Among Survivors of Intracerebral and Intraventricular Hemorrhage With Initial Severe Disability. JAMA Neurol. 79, 856–868 (2022).
Google Scholar
Zhang, Z. et al. NLRP3-dependent lipid droplet formation contributes to posthemorrhagic hydrocephalus by increasing the permeability of the blood-cerebrospinal fluid barrier in the choroid plexus. Exp. Mol. Med. 55, 574–586 (2023).
Google Scholar
Zhang, Z. et al. NLRP3 inflammasome-mediated choroid plexus hypersecretion contributes to hydrocephalus after intraventricular hemorrhage via phosphorylated NKCC1 channels. J. Neuroinflammation 19, 163 (2022).
Google Scholar
Chen, Q. et al. Post-hemorrhagic hydrocephalus: Recent advances and new therapeutic insights. J. Neurol. Sci. 375, 220–230 (2017).
Google Scholar
Ho, Y. J. et al. Effectiveness and safety of ventriculoperitoneal shunt versus lumboperitoneal shunt for communicating hydrocephalus: A systematic review and meta-analysis with trial sequential analysis. CNS Neurosci. Ther. 29, 804–815 (2023).
Google Scholar
Hanley, D. F. et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet 389, 603–611 (2017).
Google Scholar
Maller, V. V. & Gray, R. I. Noncommunicating Hydrocephalus. Semin Ultrasound CT MR 37, 109–119 (2016).
Google Scholar
Green, L. M., Wallis, T., Schuhmann, M. U. & Jaeger, M. Intracranial pressure waveform characteristics in idiopathic normal pressure hydrocephalus and late-onset idiopathic aqueductal stenosis. Fluids Barriers CNS 18, 25 (2021).
Google Scholar
Kuo, L. T. & Huang, A. P. The Pathogenesis of Hydrocephalus Following Aneurysmal Subarachnoid Hemorrhage. Int. J. Mol. Sci. 22, 5050 (2021).
Liao, F. et al. LSKL peptide alleviates subarachnoid fibrosis and hydrocephalus by inhibiting TSP1-mediated TGF-beta1 signaling activity following subarachnoid hemorrhage in rats. Exp. Ther. Med. 12, 2537–2543 (2016).
Google Scholar
Wahood, W. et al. Trends in Admissions and Outcomes for Treatment of Aneurysmal Subarachnoid Hemorrhage in the United States. Neurocrit Care 37, 209–218 (2022).
Google Scholar
Chen, Y. et al. Rethinking the initial changes in subarachnoid haemorrhage: Focusing on real-time metabolism during early brain injury. EBioMedicine 83, 104223 (2022).
Google Scholar
Duan, M. et al. Endothelial EGLN3-PKM2 signaling induces the formation of acute astrocytic barrier to alleviate immune cell infiltration after subarachnoid hemorrhage. Fluids Barriers CNS 21, 42 (2024).
Google Scholar
Sun, B. L. et al. Blocking cerebral lymphatic drainage deteriorates cerebral oxidative injury in rats with subarachnoid hemorrhage. Acta Neurochir. Suppl. 110, 49–53 (2011).
Google Scholar
Wang, Y. J. et al. The lymphatic drainage systems in the brain: a novel target for ischemic stroke? Neural Regen. Res. 18, 485–491 (2023).
Google Scholar
Perla, M., Caretti, V., Moro, M. A. & McCullough, L. D. Role of the Meningeal Lymphatics in Stroke. Stroke 54, 1670–1673 (2023).
Google Scholar
Lv, T., Zhao, B., Hu, Q. & Zhang, X. The Glymphatic System: A Novel Therapeutic Target for Stroke Treatment. Front. Aging Neurosci. 13, 689098 (2021).
Google Scholar
Koh, G. Y. & McDonald, D. M. Meningeal lymphatics can influence stroke outcome. J. Exp. Med. 221, (2024).
Ma, Q. et al. Lymphatic outflow of cerebrospinal fluid is reduced in glioma. Sci. Rep. 9, 14815 (2019).
Google Scholar
Hsu, S. J. et al. Enhanced Meningeal Lymphatic Drainage Ameliorates Neuroinflammation and Hepatic Encephalopathy in Cirrhotic Rats. Gastroenterology 160, e1313 (2021).
Google Scholar
MacDonald, M. E. et al. Lymphatic coagulation and neutrophil extracellular traps in lung-draining lymph nodes of COVID-19 decedents. Blood Adv. 6, 6249–6262 (2022).
Google Scholar
Zhang, W. et al. Coagulation in Lymphatic System. Front. Cardiovasc. Med. 8, 762648 (2021).
Google Scholar
Tambe, R. et al. Antiepileptogenic effects of borneol in pentylenetetrazole-induced kindling in mice. Naunyn Schmiedebergs Arch. Pharm. 389, 467–475 (2016).
Google Scholar
Li, W. R. et al. Pharmacokinetics of natural borneol after oral administration in mice brain and its effect on excitation ratio. Eur. J. Drug Metab. Pharmacokinet. 37, 39–44 (2012).
Google Scholar
Yu, B. et al. Effects of borneol on the pharmacokinetics of geniposide in cortex, hippocampus, hypothalamus and striatum of conscious rat by simultaneous brain microdialysis coupled with UPLC-MS. J. Pharm. Biomed. Anal. 77, 128–132 (2013).
Google Scholar
Matrongolo, M. J. et al. Piezo1 agonist restores meningeal lymphatic vessels, drainage, and brain-CSF perfusion in craniosynostosis and aged mice. J Clin Invest. 134, e171468 (2023).
Li, J. et al. Yuanzhi powder facilitated Abeta clearance in APP/PS1 mice: Target to the drainage of glymphatic system and meningeal lymphatic vessels. J. Ethnopharmacol. 319, 117195 (2024).
Google Scholar
Bec, K. B., Grabska, J. & Huck, C. W. Near-Infrared Spectroscopy in Bio-Applications. Molecules. 25, 2948 (2020).
Tao, L. et al. Microglia modulation with 1070-nm light attenuates Abeta burden and cognitive impairment in Alzheimer’s disease mouse model. Light Sci. Appl 10, 179 (2021).
Google Scholar
Baxter, G. D. et al. Low level laser therapy (Photobiomodulation therapy) for breast cancer-related lymphedema: a systematic review. BMC Cancer 17, 833 (2017).
Google Scholar
Li, D. et al. Photostimulation of lymphatic clearance of beta-amyloid from mouse brain: a new strategy for the therapy of Alzheimer’s disease. Front. Optoelectron 16, 45 (2023).
Google Scholar
Liu, S. et al. Transcranial photobiomodulation improves insulin therapy in diabetic microglial reactivity and the brain drainage system. Commun. Biol. 6, 1239 (2023).
Google Scholar
Oxana, S. G. et al. Mechanisms of phototherapy of Alzheimer’s disease during sleep and wakefulness: the role of the meningeal lymphatics. Front. Optoelectron 16, 22 (2023).
Google Scholar
Liu, Y. et al. rTMS treatment for abrogating intracerebral hemorrhage-induced brain parenchymal metabolite clearance dysfunction in male mice by regulating intracranial lymphatic drainage. Brain Behav. 13, e3062 (2023).
Google Scholar
Sachdeva, S. et al. Effects of Sound Interventions on the Permeability of the Blood-Brain Barrier and Meningeal Lymphatic Clearance. Brain Sci. 12, 742 (2022).
Murdock, M. H. et al. Multisensory gamma stimulation promotes glymphatic clearance of amyloid. Nature 627, 149–156 (2024).
Google Scholar
Hauglund, N. L., Kusk, P., Kornum, B. R. & Nedergaard, M. Meningeal Lymphangiogenesis and Enhanced Glymphatic Activity in Mice with Chronically Implanted EEG Electrodes. J. Neurosci. 40, 2371–2380 (2020).
Google Scholar
Ozturk, B. et al. Continuous positive airway pressure increases CSF flow and glymphatic transport. JCI Insight. 8, e170270 (2023).
Gao, C. et al. Craniocervical Manual Lymphatic Drainage Increases the Efficiency of Atorvastatin-Based Treatment of Chronic Subdural Hematoma. Transl. Stroke Res. 14, 667–677 (2023).
Google Scholar
Castellani, G., Croese, T., Peralta Ramos, J. M. & Schwartz, M. Transforming the understanding of brain immunity. Science 380, eabo7649 (2023).
Google Scholar
Rustenhoven, J. & Kipnis, J. Brain borders at the central stage of neuroimmunology. Nature 612, 417–429 (2022).
Google Scholar
link